Li Ka Shing Faculty of Medicine of the University of Hong Kong (HKUMed) used human respiratory organ models to investigate the infection and replication capacity of the COVID-19 Omicron BA.5 and other variants in the human respiratory tract. The results of this research reveals the mechanism behind the high infectivity and transmission ability of the BA.5 variants.
The study has been published in the Proceedings of the National Academy of Sciences.
Compared with the previous coronavirus variants, the transmissibility of the highly infectious COVID-19 Omicron variant has been ascribed to the neutralization of antibodies, resulting in immune escape. Immune escape occurs when the immune system is unable to adequately recognize and eliminate a pathogen. Thus the risk of breakthrough infection or re-infection has increased with the Omicron variant.
Scientists have considered if the evolution of the COVID-19 virus has strengthened its ability to grow and replicate in human respiratory cells, resulting in greater transmissibility, or whether Omicron variants are already well-adapted to the human respiratory cells.
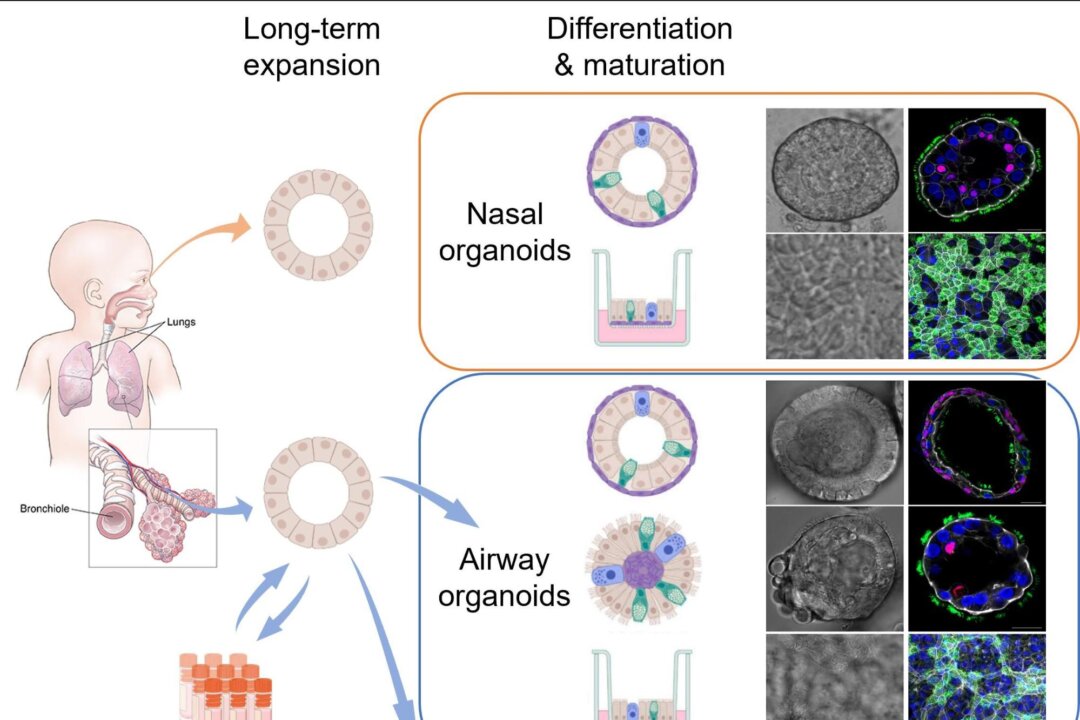
To unlock the mystery, Professor Hans Clevers from Hubrecht Institute, Dr. Zhou Jie, and Professor Yuen Kwok-yung’s research team worked together. They created the world’s first respiratory organ culture mechanism (organioid) directly from primary lung tissue and nasal cells.
An organoid is a 3D simplified and miniaturized version of an organ that is created in a lab using cells derived from human tissue, stem cells, etc. for the purpose of studying how an organ will respond and function.
Such organoid development has become the best tool for studying the biology of the respiratory tract and diseases, including COVID-19.
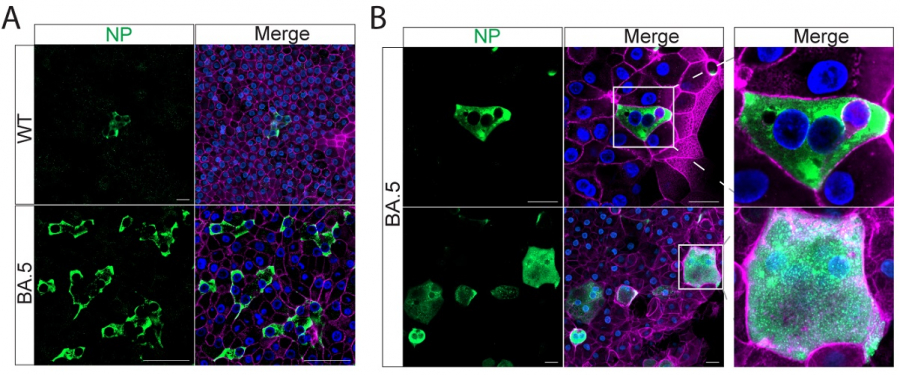
By developing stable and mature respiratory organoids with the same major attributes found in the human respiratory tract, the research team was able to observe the infection and replication of the ancestral virus strain of the two SARS-CoV-2 (WT) Omicron variants: B.1.1.529 and BA.5 in the airway and nasal mucosal organoids.
They found that the viral concentration produced after BA.5 infected human organs was significantly higher than that of the ancestral strain SARS-CoV-2 (WT) and B.1.1.529.
The replication ability reached the equivalent of influenza virus H1N1 (H1N1pdm) that led to the influenza pandemic.
The medical research team also conducted immunostaining and confocal microscopy using the infected organoids.
The number of virus-transmissible cells in BA.5-infected nasal and airway organoids was dramatically enhanced compared to the number of WT-infected and B.1.1.529-infected organoids.
The results suggested that BA.5-infected human nasal and airway epithelial cells formed prominent syncytium, while WT-infected and B.1.1.529-infected cells cannot form a syncytium. Syncytium occurs when two or more cells fuse together to form one giant cell.
In conclusion, the HKUMed research revealed a novel mechanism for the high infection transmissibility of Omicron BA.5. The development of respiratory organoids has provided a robust tool for studying respiratory biology and disease with scientific evidence that will help guide future public health policy decisions.